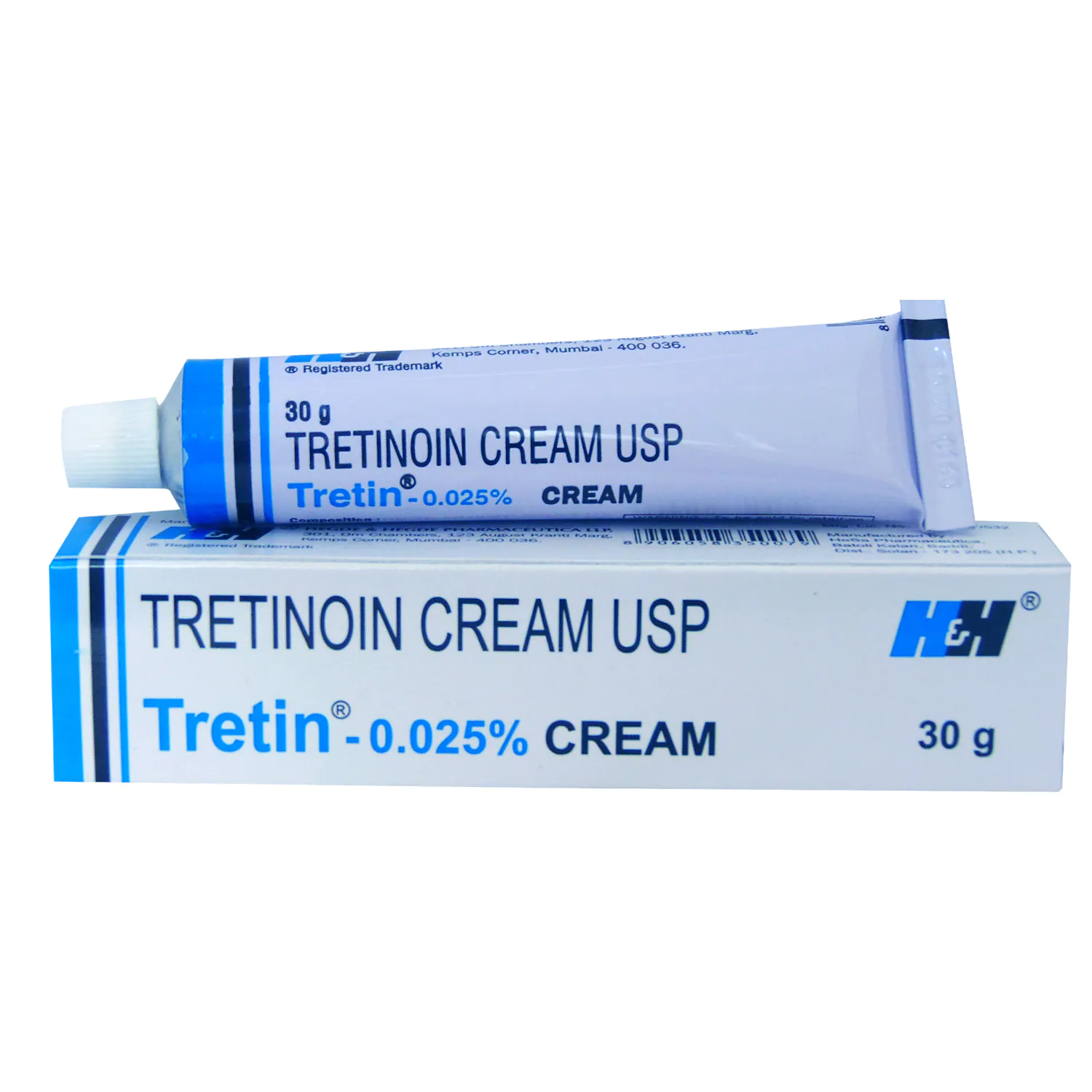
Tretinoin Cream 0.025% 30G Large Tube EXP 08/25
£28.99
Description
Tretinoin Cream 0.025% 30G Large Tube
This Tretinoin cream 0.025% is manufactured by H and H Pharmaceuticals. supplied in a large 30g aluminum tube. Tretin is a trusted brand name for tretinoin in many parts of the world, Retin-A is the brand people know in Europe and America but for large parts of the rest of the world the brand name is Tretin.
Composition. Cream base
Tretin
is a white cream.
CLINICAL PARTICULARS
Therapeutic Indications
Tretin is indicated as topical therapy for the treatment of acne vulgaris.
Posology and Method of Administration
Adults
Tretin should be applied once daily in the evening before bedtime in only
a sufficient quantity to lightly cover the entire affected areas. Prior to treatment
with Tretin areas being treated should be thoroughly cleansed with water
and a mild, non-medicated Soap. The treated area should be washed no more
than twice a day. After washing the skin should be dried gently and completely
without rubbing it. Areas of the Skin being treated should be allowed to dry for
at least 20 to 30 minutes before application of Tretin
Application of Tretin may cause a feeling of warmth and transitory
stinging. When administered according to recommended guidelines,
Tretin may produce a slight erythema similar to that of mild sunburn.
In cases where it is necessary to temporarily discontinue therapy or reduce
the frequency of application, therapy should be resumed or the frequency of
application increased when the patient becomes able to tolerate the treatment.
Frequency of application should be closely monitored by careful observation
or the clinical therapeutic response and skin tolerance
Excess application of Tretin” does not provide more rapid or better results.
In fact, marked redness, peeling or discomfort can occur. If excess application
occurs accidentally or through over-enthusiastic use, Tretin should be
discontinued for several days before resuming therapy
Therapeutic effects may be noticed after two to three weeks of use but more
than six weeks of therapy may be required before definite beneficial effects
are seen. During the early weeks of treatment, an apparent exacerbation of
inflammatory lesions may occur. This is due to the action of the medication on
deep, previously unseen lesions and should not be considered a reason, to
discontinue therapy, Once a satisfactory response has been obtained, it may
be possible to maintain this improvement with less frequent applications.
Patients treated with Tretin may use cosmetics and moisturizers, but
the areas of the skin to be treated should be cleansed thoroughly before
application of Tretin (see section on Special warnings and special
precautions for use).
Children
Safety and effectiveness have not been established in children.
Contraindications
Hypersensitivity to any component of this product.
Special Warnings and Special Precautions for Use
Local Irritation
It is not recommended to initiate treatment with Tretin® or continue its
use in the presence of skin irritation (e.g. erythema, peeling. pruritus,
sunburn, etc.) until these symptoms subside.
In certain sensitive individuals, Tretin may induce severe local
erythema, swelling, pruritus, warmth, burning or stinging, blistering, crusting
and/or peeling at the site of application. If the degree of local irritation warrants
the patient should be instructed to either apply the medication less frequently
or discontinue its use temporarily
Tretinoin has been reported to cause severe irritation on eczematous skin and
should be used with utmost caution in patients with this condition. If a patient
experiences severe or persistent irritation, the patient should be advised to
discontinue application of Tretin completely, and if necessary, consult
a physician.
In order to minimize the potential for additional skin irritation, Tretin
should be kept away from the eyes, the mouth, paranasal creases of the nose,
and mucous membranes or other areas where treatment is not intended.
Weather extremes, such as wind, cold and low humidity may be irritating to
skin treated with Tretin and may increase its dryness.
Patients will be able to remove hair as usual (e.g. plucking. electrolysis,
depilatories) but should avoid these procedures at night before applying
Tretin as they might result in skin irritation.
Permanent wave solutions, waxing preparations, medicated soaps and
Shampoos can sometimes irritate even normal skin. Caution should be used
so that these products do not come into contact with skin treated with
Tretin.
Exposure to Sunlight
Exposure to sunlight, including ultraviolet sunlamps, may provoke additional
irritation. Therefore, exposure should be avoided or minimized during the use
of Tretinoin. Patients with sunburn should be advised not to use the product
until fully recovered because of potential severe irritation to sensitive skin.
Patients who may be required to have considerable sun exposure due to their
Occupation, and those with inherent sensitivity to the sun, should exercise
particular caution. When exposure to sunlight cannot be avoided, use of
sunscreen products and protective clothing over treated areas is
recommended.
Interactions with Other Medicinal Products and Other Forms of
Interaction
The following products or medications should be used with caution because of possible interaction with Tretinoin. It is advised to allow the effects of such
preparations to subside before use of Tretin is begun:
Concomitant topical medication;
Preparations containing benzoyl peroxide, sulphur, resorcinol, or salicylic acid,
Toiletry preparations having an abrasive, drying, or desquamative effect,
including soaps, shampoos, cosmetics, and products with high
concentrations of alcohol, astringents, spices or lime
Pregnancy and Lactation
Use during pregnancy
Topical Tretinoin has not been shown to be teratogenic in Wistar rats and
rabbits when given in doses 1000 and 320 times the topical human dose,
respectively, assuming that a 50 kg adult applies 250 mg of 0.1% Tretin
cream topically. At these topical doses, however, a delayed ossification of
several bones occurred in rabbits. In rats, a dose-dependent increase of
supernumerary ribs was observed. These changes are considered variants of
normal development. The ossification changes are usually spontaneously
corrected after weaning.
There have been isolated reports of birth defects among babies born. to
women exposed to topical Tretinoin during pregnancy. To date, there have been
no adequate and well-controlled studies performed in pregnant women, and
the teratogenic blood level of Tretinoin is not known. However, a well-conducted
retrospective cohort study of babies born to women exposed to topical Tretinoin
during the first trimester of pregnancy found no excess birth defects among
these babies when compared to babies born to women in the same cohort
who were not similarly exposed. Nevertheless, topical Tretinoin should be
used during pregnancy only if the potential benefit justifies the potential risk
to the foetus (see section on Preclinical safety data).
[Oral Tretinoin has been shown to be teratogenic and fetotoxic in rats when
given in doses 2000 and 500 times the topical human dose, respectively
Whilst we guarantee original and authentic manufactured products, the content displayed here is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. We recommend that you read labels, warnings and directions of all products before use and not rely solely on the information provided
Additional information
| Weight | 0.45 kg |
|---|
45 reviews for Tretinoin Cream 0.025% 30G Large Tube EXP 08/25
Sorry, no reviews match your current selections
Related Products
-
Tretinoin Gel 0.05% 20G EXP 09/2025
Rated 4.98 out of 5£19.99 Add to cart -
My Tret Tretinoin Microsphere Gel 0.1% 15G
Rated 4.89 out of 5£19.99 Add to cart -

Azelaic Acid Cream 20% 15G Exp 05/2025
Rated 5.00 out of 5£19.99 Add to cart -

Acnelyse Tretinoin Cream 0.1% 20G EXP 11/28
Rated 5.00 out of 5£26.99 Add to cart -

Acnelyse Tretinoin Cream 0.05% 20G EXO 01/2026
Rated 5.00 out of 5£24.99 Add to cart -
Zebor Azelaic Acid Gel 20% Exp 12/24
Rated 5.00 out of 5£17.99 Add to cart -

Xtralight Tretinoin 0.05% Azelaic Acid 10% Cream 15G Exp 07/25
£21.99 Add to cart -
Revize Micro Gel 0.04% 20G Exp 09/25
Rated 5.00 out of 5£22.99 Add to cart -
Adapalene 0.1% Gel 15G Exp 08/26
£17.99 Add to cart -

20% Azelaic Acid Cream 30G Exp 01/26 Azelderm
£26.99 Add to cart

Product as described and can see a difference already
Great product and value for money
Great cream for rejuvenating your skin but ensure you read up about it first and use it correctly otherwise you could cause yourself damage.
🙏 Thank you 🫶
Everything arrived fine.